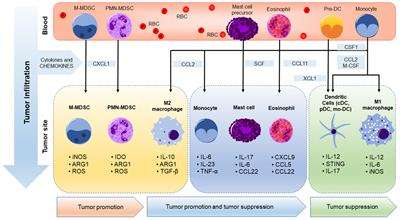
Generally, the Privacy Rule applies uniformly to all protected health information, without regard to the type of information. One exception to this general rule is for psychotherapy notes, which receive special protections. The Privacy Rule defines psychotherapy notes as notes recorded by a health care provider who is a mental health professional documenting or analyzing the contents of a conversation during a private counseling session or a group, joint, or family counseling session and that are separate from the rest of the patient’s medical record. Psychotherapy notes do not include any information about medication prescription and monitoring, counseling session start and stop times, the modalities and frequencies of treatment furnished, or results of clinical tests; nor do they include summaries of diagnosis, functional status, treatment plan, symptoms, prognosis, and progress to date. Psychotherapy notes also do not include any information that is maintained in a patient’s medical record. See 45 CFR 164.501.
Psychotherapy notes are treated differently from other mental health information both because they contain particularly sensitive information and because they are the personal notes of the therapist that typically are not required or useful for treatment, payment, or health care operations purposes, other than by the mental health professional who created the notes. Therefore, with few exceptions, the Privacy Rule requires a covered entity to obtain a patient’s authorization prior to a disclosure of psychotherapy notes for any reason, including a disclosure for treatment purposes to a health care provider other than the originator of the notes. See 45 CFR 164.508(a)(2). A notable exception exists for disclosures required by other law, such as for mandatory reporting of abuse, and mandatory “duty to warn” situations regarding threats of serious and imminent harm made by the patient (State laws vary as to whether such a warning is mandatory or permissible).
The HIPAA privacy rules give special protection to “psychotherapy notes,” but providers often misunderstand what are and are not covered and how they differ from other mental health records.
I. “Psychotherapy Notes” Defined.
Contrary to popular belief, HIPAA does not provide special protection to mental health records in general, but it does give added protection to “psychotherapy notes”. As defined by HIPAA,
Psychotherapy notes means notes recorded (in any medium) by a health care provider who is a mental health professional documenting or analyzing the contents of conversation during a private counseling session or a group, joint, or family counseling session and that are separated from the rest of the individual’s medical record. Psychotherapy notes excludes medication prescription and monitoring, counseling session start and stop times, the modalities and frequencies of treatment furnished, results of clinical tests, and any summary of the following items: Diagnosis, functional status, the treatment plan, symptoms, prognosis, and progress to date.
(45 C.F.R. § 164.501). To be considered “psychotherapy notes”, the notes must be separate from the medical record. The 2000 commentary explains the reason for this rule along with HHS’s practical view of what constitutes “psychotherapy notes”:
Comment: Some commenters thought the definition of psychotherapy notes was contrary to standard practice. They claimed that reports of psychotherapy are typically part of the medical record and that psychologists are advised, for ethical reasons and liability risk management purposes, not to keep two separate sets of notes….
Response: We conducted fact-finding with providers and other knowledgeable parties to determine the standard practice of psychotherapists and determined that only some psychotherapists keep separate files with notes pertaining to psychotherapy sessions. These notes are often referred to as “process notes,” distinguishable from “progress notes,” “the medical record,” or “official records.” These process notes capture the therapist’s impressions about the patient, contain details of the psychotherapy conversation considered to be inappropriate for the medical record, and are used by the provider for future sessions. We were told that process notes are often kept separate to limit access, even in an electronic record system, because they contain sensitive information relevant to no one other than the treating provider. These separate “process notes” are what we are calling “psychotherapy notes.” Summary information, such as the current state of the patient, symptoms, summary of the theme of the psychotherapy session, diagnoses, medications prescribed, side effects, and any other information necessary for treatment or payment, is always placed in the patient’s medical record. Information from the medical record is routinely sent to insurers for payment.
Comment: … Many commenters believed that the psychotherapy notes should include frequencies of treatment, results of clinical tests, and summary of diagnosis, functional status, the treatment plan, symptoms, prognosis and progress to date. They claimed that this information is highly sensitive and should not be released without the individual’s written consent, except in cases of emergency….
Response: As discussed above and in the NPRM, the rationale for providing special protection for psychotherapy notes is not only that they contain particularly sensitive information, but also that they are the personal notes of the therapist, intended to help him or her recall the therapy discussion and are of little or no use to others not involved in the therapy. Information in these notes is not intended to communicate to, or even be seen by, persons other than the therapist. Although all psychotherapy information may be considered sensitive, we have limited the definition of psychotherapy notes to only that information that is kept separate by the provider for his or her own purposes. It does not refer to the medical record and other sources of information that would normally be disclosed for treatment, payment, and health care operations.
Comment: One commenter was particularly concerned that the use of the term “counseling” in the definition of psychotherapy notes would lead to confusion because counseling and psychotherapy are different disciplines.
Response: In the final rule, we continue to use the term “counseling” in the definition of “psychotherapy.” During our fact-finding, we learned that “counseling” had no commonly agreed upon definition, but seemed to be widely understood in practice. We do not intend to limit the practice of psychotherapy to any specific professional disciplines.
Comment: One commenter noted that the public mental health system is increasingly being called upon to integrate and coordinate services among other providers of mental health services and they have developed an integrated electronic medical record system for state-operated hospitals, part of which includes psychotherapy notes, and which cannot be easily modified to provide different levels of confidentiality. Another commenter recommended allowing use or disclosure of psychotherapy notes by members of an integrated health care facility as well as the originator.
Response: The final rule makes it clear that any notes that are routinely shared with others, whether as part of the medical record or otherwise, are, by definition, not psychotherapy notes, as we have defined them. To qualify for the definition and the increased protection, the notes must be created and maintained for the use of the provider who created them i.e., the originator, and must not be the only source of any information that would be critical for the treatment of the patient or for getting payment for the treatment. The types of notes described in the comment would not meet our definition for psychotherapy notes.
Comment: Many providers expressed concern that if psychotherapy notes were maintained separately from other protected health information, other health providers involved in the individual’s care would be unable to treat the patient properly…
Response: The final rule retains the policy that psychotherapy notes be separated from the remainder of the medical record in order to receive additional protection. We based this decision on conversations with mental health providers who have told us that information that is critical to the treatment of individuals is normally maintained in the medical record and that psychotherapy notes are used by the provider who created them and rarely for other purposes. A strong part of the rationale for the special treatment of psychotherapy notes is that they are the personal notes of the treating provider and are of little or no use to others who were not present at the session to which the notes refer.
§ 164.508 Uses and disclosures for which an authorization is required.
(a) Standard: Authorizations for uses and disclosures –
(1) Authorization required: General rule. Except as otherwise permitted or required by this subchapter, a covered entity may not use or disclose protected health information without an authorization that is valid under this section. When a covered entity obtains or receives a valid authorization for its use or disclosure of protected health information, such use or disclosure must be consistent with such authorization.
(2) Authorization required: Psychotherapy notes. Notwithstanding any provision of this subpart, other than the transition provisions in § 164.532, a covered entity must obtain an authorization for any use or disclosure of psychotherapy notes, except:
(i) To carry out the following treatment, payment, or health care operations:
(A) Use by the originator of the psychotherapy notes for treatment;
(B) Use or disclosure by the covered entity for its own training programs in which students, trainees, or practitioners in mental health learn under supervision to practice or improve their skills in group, joint, family, or individual counseling; or
(C) Use or disclosure by the covered entity to defend itself in a legal action or other proceeding brought by the individual; and
(ii) A use or disclosure that is required by § 164.502(a)(2)(ii) or permitted by § 164.512(a); § 164.512(d) with respect to the oversight of the originator of the psychotherapy notes; § 164.512(g)(1); or § 164.512(j)(1)(i).
(3) Authorization required: Marketing.
(i) Notwithstanding any provision of this subpart, other than the transition provisions in § 164.532, a covered entity must obtain an authorization for any use or disclosure of protected health information for marketing, except if the communication is in the form of:
(A) A face-to-face communication made by a covered entity to an individual; or
(B) A promotional gift of nominal value provided by the covered entity.
(ii) If the marketing involves financial remuneration, as defined in paragraph (3) of the definition of marketing at § 164.501, to the covered entity from a third party, the authorization must state that such remuneration is involved.
(4) Authorization required: Sale of protected health information.
(i) Notwithstanding any provision of this subpart, other than the transition provisions in § 164.532, a covered entity must obtain an authorization for any disclosure of protected health information which is a sale of protected health information, as defined in § 164.501 of this subpart. (ii) Such authorization must state that the disclosure will result in remuneration to the covered entity.
(b) Implementation specifications: General requirements –
(1) Valid authorizations.
(i) A valid authorization is a document that meets the requirements in paragraphs (a)(3)(ii), (a)(4)(ii), (c)(1), and (c)(2) of this section, as applicable.
(ii) A valid authorization may contain elements or information in addition to the elements required by this section, provided that such additional elements or information are not inconsistent with the elements required by this section.
(2) Defective authorizations. An authorization is not valid, if the document submitted has any of the following defects:
(i) The expiration date has passed or the expiration event is known by the covered entity to have occurred;
(ii) The authorization has not been filled out completely, with respect to an element described by paragraph (c) of this section, if applicable;
(iii) The authorization is known by the covered entity to have been revoked;
(iv) The authorization violates paragraph (b)(3) or (4) of this section, if applicable;
(v) Any material information in the authorization is known by the covered entity to be false.
(3) Compound authorizations. An authorization for use or disclosure of protected health information may not be combined with any other document to create a compound authorization, except as follows:
(i) An authorization for the use or disclosure of protected health information for a research study may be combined with any other type of written permission for the same or another research study. This exception includes combining an authorization for the use or disclosure of protected health information for a research study with another authorization for the same research study, with an authorization for the creation or maintenance of a research database or repository, or with a consent to participate in research. Where a covered health care provider has conditioned the provision of research-related treatment on the provision of one of the authorizations, as permitted under paragraph (b)(4)(i) of this section, any compound authorization created under this paragraph must clearly differentiate between the conditioned and unconditioned components and provide the individual with an opportunity to opt in to the research activities described in the unconditioned authorization.
(ii) An authorization for a use or disclosure of psychotherapy notes may only be combined with another authorization for a use or disclosure of psychotherapy notes.
(iii) An authorization under this section, other than an authorization for a use or disclosure of psychotherapy notes, may be combined with any other such authorization under this section, except when a covered entity has conditioned the provision of treatment, payment, enrollment in the health plan, or eligibility for benefits under paragraph (b)(4) of this section on the provision of one of the authorizations. The prohibition in this paragraph on combining authorizations where one authorization conditions the provision of treatment, payment, enrollment in a health plan, or eligibility for benefits under paragraph (b)(4) of this section does not apply to a compound authorization created in accordance with paragraph (b)(3)(i) of this section.
(4) Prohibition on conditioning of authorizations. A covered entity may not condition the provision to an individual of treatment, payment, enrollment in the health plan, or eligibility for benefits on the provision of an authorization, except:
(i) A covered health care provider may condition the provision of research-related treatment on provision of an authorization for the use or disclosure of protected health information for such research under this section;
(ii) A health plan may condition enrollment in the health plan or eligibility for benefits on provision of an authorization requested by the health plan prior to an individual’s enrollment in the health plan, if:
(A) The authorization sought is for the health plan’s eligibility or enrollment determinations relating to the individual or for its underwriting or risk rating determinations; and
(B) The authorization is not for a use or disclosure of psychotherapy notes under paragraph (a)(2) of this section; and
(iii) A covered entity may condition the provision of health care that is solely for the purpose of creating protected health information for disclosure to a third party on provision of an authorization for the disclosure of the protected health information to such third party.
(5) Revocation of authorizations. An individual may revoke an authorization provided under this section at any time, provided that the revocation is in writing, except to the extent that:
(i) The covered entity has taken action in reliance thereon; or
(ii) If the authorization was obtained as a condition of obtaining insurance coverage, other law provides the insurer with the right to contest a claim under the policy or the policy itself.
(6) Documentation. A covered entity must document and retain any signed authorization under this section as required by § 164.530(j).
(c) Implementation specifications: Core elements and requirements –
(1) Core elements. A valid authorization under this section must contain at least the following elements:
(i) A description of the information to be used or disclosed that identifies the information in a specific and meaningful fashion.
(ii) The name or other specific identification of the person(s), or class of persons, authorized to make the requested use or disclosure.
(iii) The name or other specific identification of the person(s), or class of persons, to whom the covered entity may make the requested use or disclosure.
(iv) A description of each purpose of the requested use or disclosure. The statement “at the request of the individual” is a sufficient description of the purpose when an individual initiates the authorization and does not, or elects not to, provide a statement of the purpose.
(v) An expiration date or an expiration event that relates to the individual or the purpose of the use or disclosure. The statement “end of the research study,” “none,” or similar language is sufficient if the authorization is for a use or disclosure of protected health information for research, including for the creation and maintenance of a research database or research repository.
(vi) Signature of the individual and date. If the authorization is signed by a personal representative of the individual, a description of such representative’s authority to act for the individual must also be provided.
(2) Required statements. In addition to the core elements, the authorization must contain statements adequate to place the individual on notice of all of the following:
(i) The individual’s right to revoke the authorization in writing, and either:
(A) The exceptions to the right to revoke and a description of how the individual may revoke the authorization; or
(B) To the extent that the information in paragraph (c)(2)(i)(A) of this section is included in the notice required by § 164.520, a reference to the covered entity’s notice.
(ii) The ability or inability to condition treatment, payment, enrollment or eligibility for benefits on the authorization, by stating either:
(A) The covered entity may not condition treatment, payment, enrollment or eligibility for benefits on whether the individual signs the authorization when the prohibition on conditioning of authorizations in paragraph (b)(4) of this section applies; or
(B) The consequences to the individual of a refusal to sign the authorization when, in accordance with paragraph (b)(4) of this section, the covered entity can condition treatment, enrollment in the health plan, or eligibility for benefits on failure to obtain such authorization.
(iii) The potential for information disclosed pursuant to the authorization to be subject to redisclosure by the recipient and no longer be protected by this subpart.
(3) Plain language requirement. The authorization must be written in plain language.
(4) Copy to the individual. If a covered entity seeks an authorization from an individual for a use or disclosure of protected health information, the covered entity must provide the individual with a copy of the signed authorization.