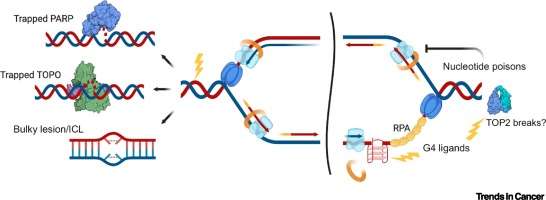
-
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
-
Zeman, M. K. & Cimprich, K. A. Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9 (2014).
-
Gaillard, H., García-Muse, T. & Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer 15, 276–280 (2015).
-
Halazonetis, T. D., Gorgoulis, V. G. & Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 319, 1352–1355 (2008).
-
Wilhelm, T., Said, M. & Naim, V. DNA replication stress and chromosomal instability: dangerous liaisons. Genes 11, 1–35 (2020).
-
O’Connor, M. J. Targeting the DNA damage response in cancer. Mol. Cell 60, 547–560 (2015).
-
Cimprich, K. A., Shin, T. B., Keith, C. T. & Schreiber, S. L. cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc. Natl Acad. Sci. USA 93, 2850–2855 (1996). This paper is the first to describe the protein encoded by the ATR gene, then called FRAP-related protein, and considered the human counterpart of known essential proteins for S and G2/M checkpoints in other species.
-
Bentley, N. J. et al. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBOJ 15, 6641–6651 (1996).
-
Dobbelstein, M. & Sørensen, C. S. Exploiting replicative stress to treat cancer. Nat. Rev. Drug. Discov. 14, 405–423 (2015).
-
Bell, D. et al. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
-
Lecona, E. & Fernandez-Capetillo, O. Targeting ATR in cancer. Nat. Rev. Cancer 18, 586–595 (2018).
-
Karnitz, L. M. & Zou, L. Molecular pathways: targeting ATR in cancer therapy. Clin. Cancer Res. 21, 4780–4785 (2015).
-
Saldivar, J. C., Cortez, D. & Cimprich, K. A. The essential kinase ATR: ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 18, 622–636 (2017).
-
Quinet, A., Tirman, S., Cybulla, E., Meroni, A. & Vindigni, A. To skip or not to skip: choosing repriming to tolerate DNA damage. Mol. Cell 81, 649–658 (2021).
-
Pfister, S. X. et al. Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Cancer Cell 28, 557–568 (2015).
-
Domínguez-Kelly, R. et al. Wee1 controls genomic stability during replication by regulating the Mus81-Eme1 endonuclease. J. Cell Biol. 194, 567–579 (2011).
-
Martin, J. C. et al. Exploiting replication stress as a novel therapeutic intervention. Mol. Cancer Res. 19, 192–206 (2020).
-
Coschi, C. H. et al. Haploinsufficiency of an RB-E2F1-condensin II complex leads to aberrant replication and aneuploidy. Cancer Discov. 4, 840–853 (2014).
-
Schoonen, P. M., Guerrero Llobet, S. & van Vugt, M. A. T. M. Replication stress: driver and therapeutic target in genomically instable cancers. Adv. Protein Chem. Struct. Biol. 115, 157–201 (2019).
-
Macheret, M. & Halazonetis, T. D. Intragenic origins due to short G1 phases underlie oncogene-induced DNA replication stress. Nature 555, 112–116 (2018).
-
Bartkova, J. et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 (2006).
-
Nazareth, D., Jones, M. J. K. & Gabrielli, B. Everything in moderation: lessons learned by exploiting moderate replication stress in cancer. Cancers 11, 1320 (2019).
-
Medda, A., Duca, D. & Chiocca, S. Human papillomavirus and cellular pathways: hits and targets. Pathogens 10, 262 (2021).
-
Rottenberg, S., Disler, C. & Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 21, 37–50 (2021).
-
Zhu, H., Swami, U., Preet, R. & Zhang, J. Harnessing DNA replication stress for novel cancer therapy. Genes 11, 990 (2020).
-
Gralewska, P. et al. PARP inhibition increases the reliance on ATR/CHK1 checkpoint signaling leading to synthetic lethality — an alternative treatment strategy for epithelial ovarian cancer cells independent from HR effectiveness. Int. J. Mol. Sci. 21, 9715 (2020).
-
Liao, H., Ji, F., Helleday, T. & Ying, S. Mechanisms for stalled replication fork stabilization: new targets for synthetic lethality strategies in cancer treatments. EMBO Rep. 19, e46263 (2018).
-
Huang, T. T. et al. Targeting the PI3K/mTOR pathway augments CHK1 inhibitor-induced replication stress and antitumor activity in high-grade serous ovarian cancer. Cancer Res. 80, 5380–5392 (2020).
-
Flem-Karlsen, K. et al. Targeting AXL and the DNA damage response pathway as a novel therapeutic strategy in melanoma. Mol. Cancer Ther. 19, 895–905 (2020).
-
Ramkumar, K. et al. AXL inhibition induces DNA damage and replication stress in non-small cell lung cancer cells and promotes sensitivity to ATR inhibitors. Mol. Cancer Res. 19, 485–497 (2021).
-
Yan, D., Shelton Earp, H., DeRyckere, D. & Graham, D. K. Targeting MERTK and AXL in EGFR mutant non-small cell lung cancer. Cancers 13, 5639 (2021).
-
McDaniel, N. K. et al. AXL mediates cetuximab and radiation resistance through tyrosine 821 and the c-abl kinase pathway in head and neck cancer. Clin. Cancer Res. 26, 4349–4359 (2020).
-
Bowry, A. & Kelly, R. D. W. Hypertranscription and replication stress in cancer. Trends Cancer 7, 863-877 (2021).
-
Zhang, J. et al. BRD4 facilitates replication stress-induced DNA damage response. Oncogene 37, 3763–3777 (2018).
-
Zhang, P. et al. BRD4 inhibitor AZD5153 suppresses the proliferation of colorectal cancer cells and sensitizes the anticancer effect of PARP inhibitor. Int. J. Biol. Sci. 15, 1942–1954 (2019).
-
Takashima, Y. et al. Bromodomain and extraterminal domain inhibition synergizes with WEE1-inhibitor AZD1775 effect by impairing nonhomologous end joining and enhancing DNA damage in nonsmall cell lung cancer. Int. J. Cancer 146, 1114–1124 (2020).
-
Muralidharan, S. V. et al. BET bromodomain inhibitors synergize with ATR inhibitors to induce DNA damage, apoptosis, senescence-associated secretory pathway and ER stress in Myc-induced lymphoma cells. Oncogene 35, 4689–4697 (2016).
-
Nayak, S. et al. Inhibition of the translesion synthesis polymerase REV1 exploits replication gaps as a cancer vulnerability. Sci. Adv. 6, eaaz7808 (2020).
-
Kim, W. et al. USP13 regulates the replication stress response by deubiquitinating TopBP1. DNA Repair 100, 103063 (2021).
-
Yu, X. et al. Ubiquitination of the DNA-damage checkpoint kinase CHK1 by TRAF4 is required for CHK1 activation. J. Hematol. Oncol. 13, 1–19 (2020).
-
D’Andrea, A. D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair 71, 172–176 (2018).
-
Lee, E. K. & Matulonis, U. A. Parp inhibitor resistance mechanisms and implications for post-progression combination therapies. Cancers 12, 1–25 (2020).
-
Peng, Y. et al. The deubiquitylating enzyme USP15 regulates homologous recombination repair and cancer cell response to PARP inhibitors. Nat. Commun. 10, 1224 (2019).
-
Gogola, E. et al. Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell 33, 1078–1093.e12 (2018).
-
Hill, S. J. et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 8, 1404–1421 (2018). This seminal paper on ovarian cancer organoids shows the utility of these preclinical models to assess drug sensitivity and perform functional assays such as the DNA combining assay to assess the level and response to replication stress in patient tumour samples.
-
Kim, H. et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 11, 3726 (2020).
-
Dréan, A. et al. Modeling therapy resistance in BRCA1/2-mutant cancers. Mol. Cancer Ther. 16, 2022–2034 (2017).
-
Parmar, K. et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin. Cancer Res. 25, 6127–6140 (2019). This paper shows the monotherapy activity of the CHK1 inhibitor prexasertib in in vitro and in vivo preclinical models of PARPi-resistant ovarian cancer by promoting homologous recombination deficiency and replication fork instability.
-
Yazinski, S. A. et al. ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes Dev. 31, 318–332 (2017). This is a comprehensive preclinical study evaluating the role of ATR inhibition in overcoming PARPi resistance in BRCA1-deficient cells by promoting homologous recombination deficiency and replication fork instability.
-
Murai, J. et al. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 7, 76534–76550 (2016).
-
Ha, D.-H. et al. Antitumor effect of a WEE1 inhibitor and potentiation of olaparib sensitivity by DNA damage response modulation in triple-negative breast cancer. Sci. Rep. 10, 9930 (2020).
-
Moens, S. et al. The mitotic checkpoint is a targetable vulnerability of carboplatin-resistant triple negative breast cancers. Sci. Rep. 11, 3176 (2021).
-
Shi, Q. et al. The identification of the ATR inhibitor VE-822 as a therapeutic strategy for enhancing cisplatin chemosensitivity in esophageal squamous cell carcinoma. Cancer Lett. 432, 56–68 (2018).
-
Hall, A. B. et al. Potentiation of tumor responses to DNA damaging therapy by the selective ATR inhibitor VX-970. Oncotarget 5, 5674–5685 (2014).
-
Leonard, B. C. et al. ATR inhibition sensitizes HPV− and HPV+ head and neck squamous cell carcinoma to cisplatin. Oral. Oncol. 95, 35–42 (2019).
-
Pillay, N. et al. DNA replication vulnerabilities render ovarian cancer cells sensitive to poly(ADP-ribose) glycohydrolase inhibitors. Cancer Cell 35, 519–533.e8 (2019).
-
Min, W. et al. Poly(ADP-ribose) binding to CHK1 at stalled replication forks is required for S-phase checkpoint activation. Nat. Commun. 4, 2993 (2013).
-
Murai, J., Thomas, A., Miettinen, M. & Pommier, Y. Schlafen 11 (SLFN11), a restriction factor for replicative stress induced by DNA-targeting anti-cancer therapies. Pharmacol. Ther. 201, 94–102 (2019).
-
Mao, S. et al. Resistance to pyrrolobenzodiazepine dimers is associated with SLFN11 downregulation and can be reversed through inhibition of ATR. Mol. Cancer Ther. 20, 541–552 (2021).
-
Winkler, C. et al. SLFN11 informs on standard of care and novel treatments in a wide range of cancer models. Br. J. Cancer 124, 951–962 (2021).
-
Shen, J. et al. PARPI triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCANEss. Cancer Res. 79, 311–319 (2019).
-
Ding, L. et al. PARP inhibition elicits STING-dependent antitumor immunity in BRCA1-deficient ovarian cancer. Cell Rep. 25, 2972–2980.e5 (2018). This is one of the first studies to show that PARPi activate the cGAS–STING pathway to promote antitumour immunity.
-
Schoonen, P. M. et al. Premature mitotic entry induced by ATR inhibition potentiates olaparib inhibition-mediated genomic instability, inflammatory signaling, and cytotoxicity in BRCA2-deficient cancer cells. Mol. Oncol. 13, 2422–2440 (2019).
-
Mouw, K. W. & Konstantinopoulos, P. A. From checkpoint to checkpoint: DNA damage ATR/Chk1 checkpoint signalling elicits PD-L1 immune checkpoint activation. Br. J. Cancer 118, 933–935 (2018).
-
Sato, H. et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 8, 1751 (2017).
-
Sun, L.-L. et al. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am. J. Cancer Res. 8, 1307–1316 (2018).
-
Wayne, J., Brooks, T., Landras, A. & Massey, A. J. Targeting DNA damage response pathways to activate the STING innate immune signaling pathway in human cancer cells. FEBS J. 288, 4507–4540 (2021).
-
Patel, P. et al. Enhancing direct cytotoxicity and response to immune checkpoint blockade following ionizing radiation with Wee1 kinase inhibition. Oncoimmunology 8, e1638207 (2019).
-
Sen, T. et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 9, 646–661 (2019).
-
Sen, T. et al. Combination treatment of the oral CHK1 inhibitor, SRA737, and low-dose gemcitabine enhances the effect of programmed death ligand 1 blockade by modulating the immune microenvironment in SCLC. J. Thorac. Oncol. 14, 2152–2163 (2019).
-
Alimzhanov, M. et al. Abstract 2269: ATR inhibitor M6620 enhances anti-tumor efficacy of the combination of the anti-PD-L1 antibody avelumab with platinum-based chemotherapy. Cancer Res. 79 (Suppl. 13), Abstr. 2269 (2019).
-
Zhang, S. et al. Genetically defined, syngeneic organoid platform for developing combination therapies for ovarian cancer. Cancer Discov. 11, 362–383 (2021).
-
Iyer, S. et al. Genetically defined syngeneic mouse models of ovarian cancer as tools for the discovery of combination immunotherapy. Cancer Discov. 11, 384–407 (2021).
-
Tang, Z. et al. ATR inhibition induces CDK1–SPOP signaling and enhances anti-PD-L1 cytotoxicity in prostate cancer. Clin. Cancer Res. 27, 4898–4909 (2021).
-
Pilger, D., Seymour, L. W. & Jackson, S. P. Interfaces between cellular responses to DNA damage and cancer immunotherapy. Genes. Dev. 35, 602–618 (2021).
-
Sheng, H. et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J. Immunother. Cancer 8, e000340 (2020).
-
Young, L. A. et al. Differential activity of ATR and Wee1 inhibitors in a highly sensitive subpopulation of DLBCL linked to replication stress. Cancer Res. 79, 3762–3775 (2019).
-
Aarts, M. et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov. 2, 524–539 (2012).
-
Moiseeva, T. N., Qian, C., Sugitani, N., Osmanbeyoglu, H. U. & Bakkenist, C. J. WEE1 kinase inhibitor AZD1775 induces CDK1 kinase-dependent origin firing in unperturbed G1- and S-phase cells. Proc. Natl Acad. Sci. USA 116, 23891–23893 (2019).
-
Beck, H. et al. Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol. Cell Biol. 32, 4226–4236 (2012).
-
Cuneo, K. C. et al. Wee1 kinase inhibitor AZD1775 radiosensitizes hepatocellular carcinoma regardless of TP53 mutational status through induction of replication stress. Int. J. Radiat. Oncol. Biol. Phys. 95, 782–790 (2016).
-
King, C. et al. LY2606368 causes replication catastrophe and antitumor effects through CHK1-dependent mechanisms. Mol. Cancer Ther. 14, 2004–2013 (2015).
-
Morgan, M. A. et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 70, 4972–4981 (2010).
-
Dibitetto, D. et al. Intrinsic ATR signaling shapes DNA end resection and suppresses toxic DNA-PKcs signaling. Nar. Cancer 2, 1–14 (2020).
-
Sørensen, C. S. et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 7, 195–201 (2005). This is the first study to propose that CHK1 is not only crucial for cell-cycle regulation but also has a fundamental role in DNA repair.
-
Pefani, D. E. et al. RASSF1A-LATS1 signalling stabilizes replication forks by restricting CDK2-mediated phosphorylation of BRCA2. Nat. Cell Biol. 16, 962–971 (2014).
-
Parsels, L. A. et al. Gemcitabine sensitization by checkpoint kinase 1 inhibition correlates with inhibition of a Rad51 DNA damage response in pancreatic cancer cells. Mol. Cancer Ther. 8, 45–54 (2009).
-
Kausar, T. et al. Sensitization of pancreatic cancers to gemcitabine chemoradiation by WEE1 kinase inhibition depends on homologous recombination repair. Neoplasia 17, 757–766 (2015).
-
Aarts, M. et al. Functional genetic screen identifies increased sensitivity to WEE1 inhibition in cells with defects in Fanconi anemia and HR pathways. Mol. Cancer Ther. 14, 865–876 (2015).
-
Guertin, A. D. et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol. Cancer Ther. 12, 1442–1452 (2013).
-
Kreahling, J. M. et al. MK1775, a selective WEE1 inhibitor, shows single-agent antitumor activity against sarcoma cells. Mol. Cancer Ther. 11, 174–182 (2012).
-
Reaper, P. M. et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat. Chem. Biol. 7, 428–430 (2011).
-
Hirai, H. et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol. Cancer Ther. 8, 2992–3000 (2009).
-
Bridges, K. A. et al. MK-1775, a novel Wee1 kinase inhibitor,radiosensitizes p53-defective human tumor cells. Clin. Cancer Res. 17, 5638–5648 (2011).
-
Pappano, W. N., Zhang, Q., Tucker, L. A., Tse, C. & Wang, J. Genetic inhibition of the atypical kinase Wee1 selectively drives apoptosis of p53 inactive tumor cells. BMC Cancer 14, 430 (2014).
-
Dillon, M. T. et al. Radiosensitization by the ATR inhibitor AZD6738 through generation of acentric micronuclei. Mol. Cancer Ther. 16, 25–34 (2017).
-
Middleton, F. K., Pollard, J. R. & Curtin, N. J. The impact of p53 dysfunction in ATR inhibitor cytotoxicity and chemo- and radiosensitisation. Cancers 10, 275 (2018).
-
Van Linden, A. A. et al. Inhibition of wee1 sensitizes cancer cells to antimetabolite chemotherapeutics in vitro and in vivo, independent of p53 functionality. Mol. Cancer Ther. 12, 2675–2684 (2013).
-
Dillon, M. et al. A phase I study of ATR inhibitor, AZD6738, as monotherapy in advanced solid tumours (PATRIOT part A, B). Ann. Oncol. 30, v165–v166 (2019).
-
Aggarwal, R. et al. 512O. Interim results from a phase II study of the ATR inhibitor ceralasertib in ARID1A-deficient and ARID1A-intact advanced solid tumor malignancies. Ann. Oncol. 32, S583 (2021).
-
Yap, T. A. et al. First-in-human trial of the oral ataxia telangiectasia and Rad3-related inhibitor BAY 1895344 in patients with advanced solid tumors. Cancer Discov. 11, 80–91 (2021).
-
Yap, T. A. et al. Phase I trial of first-in-class ATR inhibitor M6620 (VX-970) as monotherapy or in combination with carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 38, 3195–3204 (2020).
-
Yap, T. A. et al. Genomic and pathologic determinants of response to RP-3500, an ataxia telangiectasia and Rad3-related inhibitor (ATRi), in patients (pts) with DNA damage repair (DDR) loss-of-function (LOF) mutant tumors in the phase 1/2 TRESR trial. Cancer Res. 82 (Suppl. 12), Abstr. CT030 (2022).
-
Daud, A. I. et al. Phase I dose-escalation trial of checkpoint kinase 1 inhibitor MK-8776 as monotherapy and in combination with gemcitabine in patients with advanced solid tumors. J. Clin. Oncol. 33, 1060–1066 (2015).
-
Italiano, A. et al. Phase I study of the checkpoint kinase 1 inhibitor GDC-0575 in combination with gemcitabine in patients with refractory solid tumors. Ann. Oncol. 29, 1304–1311 (2018).
-
Laquente, B. et al. A phase II study to evaluate LY2603618 in combination with gemcitabine in pancreatic cancer patients. BMC Cancer 17, 137 (2017).
-
Scagliotti, G. et al. Phase II evaluation of LY2603618, a first-generation CHK1 inhibitor, in combination with pemetrexed in patients with advanced or metastatic non-small cell lung cancer. Invest. New Drugs 34, 625–635 (2016).
-
Doi, T. et al. Phase I study of LY2603618, a CHK1 inhibitor, in combination with gemcitabine in Japanese patients with solid tumors. Anticancer Drugs 26, 1043–1053 (2015).
-
Calvo, E. et al. Phase I study of CHK1 inhibitor LY2603618 in combination with gemcitabine in patients with solid tumors. Oncology 91, 251–260 (2016).
-
Seto, T. et al. Phase I, dose-escalation study of AZD7762 alone and in combination with gemcitabine in Japanese patients with advanced solid tumours. Cancer Chemother. Pharmacol. 72, 619–627 (2013).
-
Sausville, E. et al. Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother. Pharmacol. 73, 539–549 (2014).
-
Hong, D. et al. Phase i study of LY2606368, a checkpoint kinase 1 inhibitor, in patients with advanced cancer. J. Clin. Oncol. 34, 1764–1771 (2016).
-
Hong, D. S. et al. Evaluation of prexasertib, a checkpoint kinase 1 inhibitor, in a phase Ib study of patients with squamous cell carcinoma. Clin. Cancer Res. 24, 3263–3272 (2018).
-
Lee, J. M. et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. Lancet Oncol. 19, 207–215 (2018). This clinical study is the first to show the activity of a CHK1 inhibitor to treat HGSOC.
-
Lampert, E. J. et al. Prexasertib, a cell cycle checkpoint kinase 1 inhibitor, in BRCA mutant recurrent high-grade serous ovarian cancer (HGSOC): a proof-of-concept single arm phase II study. J. Clin. Oncol. 38, 6038 (2020).
-
Ditano, J. P. & Eastman, A. comparative activity and off-target effects in cells of the CHK1 inhibitors MK-8776, SRA737, and LY2606368. ACS Pharmacol. Transl. Sci. 4, 730–743 (2021).
-
Plummer, E. R. et al. A first-in-human phase I/II trial of SRA737 (a Chk1 Inhibitor) in subjects with advanced cancer. J. Clin. Oncol. 37, 3094 (2019).
-
Miller, W. H. et al. A phase Ib study of oral Chk1 inhibitor LY2880070 as monotherapy in patients with advanced or metastatic cancer. J. Clin. Oncol. 38, 3579 (2020).
-
Do, K. et al. Phase I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor, in patients with refractory solid tumors. J. Clin. Oncol. 33, 3409–3415 (2015).
-
Takebe, N. et al. Safety, antitumor activity, and biomarker analysis in a phase I trial of the once-daily wee1 inhibitor adavosertib (AZD1775) in patients with advanced solid tumors. Clin. Cancer Res. 27, 3834–3844 (2021).
-
Tolcher, A. et al. Clinical activity of single-agent ZN-c3, an oral WEE1 inhibitor, in a phase 1 dose-escalation trial in patients with advanced solid tumors. Cancer Res. 81 (Suppl. 13), Abstr. CT016 (2021).
-
Pasic, A. et al. A phase 1b dose-escalation study of ZN-c3, a WEE1 inhibitor, in combination with chemotherapy (CT) in subjects with platinum-resistant or refractory ovarian, peritoneal, or fallopian tube cancer. Cancer Res. 82 (Suppl. 12), Abstr. CT148 (2022).
-
Lheureux, S. et al. Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 281–292 (2021). This is one of the two main randomized trials showing activity of the combination of a WEE1i with gemcitabine in platinum-resistant ovarian cancers.
-
Meric-Bernstam, F. et al. Safety and clinical activity of single-agent ZN-c3, an oral WEE1 inhibitor, in a phase 1 trial in subjects with recurrent or advanced uterine serous carcinoma (USC). Cancer Res. 82 (Suppl. 12), Abstr. CT029 (2022).
-
Fu, S. et al. Phase II trial of the Wee1 inhibitor adavosertib in advanced refractory solid tumors with CCNE1 amplification. Cancer Res. 81 (Suppl. 13), Abstr. 974 (2021).
-
Ghelli Luserna Di Rorà, A., Cerchione, C., Martinelli, G. & Simonetti, G. A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target. J. Hematol. Oncol. 13, 126 (2020).
-
Gallo, D. et al. CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature 604, 749–756 (2022). This is the first preclinical study to show the synthetic lethality of PKMYT1 inhibition with CCNE1 amplification.
-
Meng, X. et al. AZD1775 increases sensitivity to olaparib and gemcitabine in cancer cells with p53 mutations. Cancers 10, 149 (2018).
-
Xiao, Y. et al. Identification of preferred chemotherapeutics for combining with a CHK1 Inhibitor. Mol. Cancer Ther. 12, 2285–2295 (2013).
-
Kreahling, J. M. et al. Wee1 inhibition by MK-1775 leads to tumor inhibition and enhances efficacy of gemcitabine in human sarcomas. PLoS ONE 8, e57523 (2013).
-
Koh, S. B. et al. Mechanistic distinctions between CHK1 and WEE1 inhibition guide the scheduling of triple therapy with gemcitabine. Cancer Res. 78, 3054–3066 (2018).
-
Montano, R., Chung, I., Garner, K. M., Parry, D. & Eastman, A. Preclinical development of the novel Chk1 inhibitor SCH900776 in combination with DNA-damaging agents and antimetabolites. Mol. Cancer Ther. 11, 427–438 (2012).
-
Hirai, H. et al. MK-1775, a small molecule Wee1 inhibitor, enhances antitumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol. Ther. 9, 514–522 (2010).
-
Fordham, S. E. et al. Inhibition of ATR acutely sensitizes acute myeloid leukemia cells to nucleoside analogs that target ribonucleotide reductase. Blood Adv. 2, 1157–1169 (2018).
-
Liu, S. et al. Inhibition of ATR potentiates the cytotoxic effect of gemcitabine on pancreatic cancer cells through enhancement of DNA damage and abrogation of ribonucleotide reductase induction by gemcitabine. Oncol. Rep. 37, 3377–3386 (2017).
-
Wallez, Y. et al. The ATR inhibitor AZD6738 synergizes with gemcitabine in vitro and in vivo to induce pancreatic ductal adenocarcinoma regression. Mol. Cancer Ther. 17, 1670–1682 (2018).
-
Venkatesha, V. A. et al. Sensitization of pancreatic cancer stem cells to gemcitabine by Chk1 inhibition. Neoplasia 14, 519–525 (2012).
-
Warren, N. J. H. & Eastman, A. Inhibition of checkpoint kinase 1 following gemcitabine-mediated S phase arrest results in CDC7- and CDK2-dependent replication catastrophe. J. Biol. Chem. 294, 1763–1778 (2019).
-
Montano, R. et al. Sensitization of human cancer cells to gemcitabine by the Chk1 inhibitor MK-8776: cell cycle perturbation and impact of administration schedule in vitro and in vivo. BMC Cancer 13, 604 (2013).
-
Leijen, S. et al. Phase I study evaluating WEE1 inhibitor AZD1775 as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 34, 4371–4380 (2016). This was one of the first clinical studies showing the safety and activity of WEE inhibition in patients with solid tumors.
-
Cuneo, K. C. et al. Dose escalation trial of the WEE1 inhibitor adavosertib (AZD1775) in combination with gemcitabine and radiation for patients with locally advanced pancreatic cancer. J. Clin. Oncol. 37, 2643–2650 (2019).
-
Banerji, U. et al. A phase I/II first-in-human trial of oral SRA737 (a Chk1 inhibitor) given in combination with low-dose gemcitabine in subjects with advanced cancer. J. Clin. Oncol. 37, 3095 (2019).
-
Hong, D. S. et al. Preclinical evaluation and phase Ib study of prexasertib, a CHK1 inhibitor, and samotolisib (LY3023414), a dual PI3K/mTOR inhibitor. Clin. Cancer Res. 27, 1864–1874 (2021).
-
Konstantinopoulos, P. A. et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 21, 957–968 (2020). This is the first randomized study to show the activity of the ATRi berzosertib in patients with platinum-resistant HGSOC.
-
Leijen, S. et al. Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J. Clin. Oncol. 34, 4354–4361 (2016).
-
Oza, A. M. et al. A biomarker-enriched, randomized phase II trial of adavosertib (AZD1775) plus paclitaxel and carboplatin for women with platinum-sensitive TP53-mutant ovarian cancer. Clin. Cancer Res. 26, 4767–4776 (2020).
-
Moore, K. N. et al. Adavosertib with chemotherapy in patients with primary platinum-resistant ovarian, fallopian tube, or peritoneal cancer: an open-label, four-arm, phase II study. Clin. Cancer Res. 28, 36–44 (2022).
-
Huntoon, C. J. et al. ATR inhibition broadly sensitizes ovarian cancer cells to chemotherapy independent of BRCA status. Cancer Res. 73, 3683–3691 (2013).
-
Zheng, H., Shao, F., Martin, S., Xu, X. & Deng, C. X. WEE1 inhibition targets cell cycle checkpoints for triple negative breast cancers to overcome cisplatin resistance. Sci. Rep. 7, 43517 (2017).
-
Jhuraney, A. et al. PAXIP1 potentiates the combination of WEE1 inhibitor AZD1775 and platinum agents in lung cancer. Mol. Cancer Ther. 15, 1669–1681 (2016).
-
Osman, A. A. et al. Wee-1 kinase inhibition overcomes cisplatin resistance associated with high-risk TP53 mutations in head and neck cancer through mitotic arrest followed by senescence. Mol. Cancer Ther. 14, 608–619 (2015).
-
Mendez, E. et al. A phase I clinical trial of AZD1775 in combination with neoadjuvant weekly docetaxel and cisplatin before definitive therapy in head and neck squamous cell carcinoma. Clin. Cancer Res. 24, 2740–2748 (2018).
-
Yap, T. A. et al. Ceralasertib (AZD6738), an oral ATR kinase inhibitor, in combination with carboplatin in patients with advanced solid tumors: a phase I study. Clin. Cancer Res. 27, 5213–5224 (2021).
-
Thomas, A. et al. Phase I study of ATR inhibitor M6620 in combination with topotecan in patients with advanced solid tumors. J. Clin. Oncol. 36, 1594–1602 (2018).
-
Kabeche, L., Nguyen, H. D., Buisson, R. & Zou, L. A mitosis-specific and R loop–driven ATR pathway promotes faithful chromosome segregation. Science 359, 108–114 (2018).
-
Kim, S. T. et al. Phase I study of ceralasertib (AZD6738), a novel DNA damage repair agent, in combination with weekly paclitaxel in refractory cancer. Clin. Cancer Res. 27, 4700–4709 (2021).
-
Kim, H. et al. Targeting the ATR/CHK1 axis with PARP inhibition results in tumor regression in BRCA-mutant ovarian cancer models. Clin. Cancer Res. 23, 3097–3108 (2017).
-
Fang, Y. et al. Sequential therapy with PARP and WEE1 inhibitors minimizes toxicity while maintaining efficacy. Cancer Cell 35, 851–867.e7 (2019). This study proposes an innovative schedule for the combination of PARPi and WEEi to overcome the toxicity of the combination.
-
Burgess, B. T. et al. Olaparib combined with an ATR or Chk1 inhibitor as a treatment strategy for acquired olaparib-resistant BRCA1 mutant ovarian cells. Diagnostics 10, 121 (2020).
-
Brill, E. et al. Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer. Oncotarget 8, 111026–111040 (2017).
-
Mani, C. et al. Prexasertib treatment induces homologous recombination deficiency and synergizes with olaparib in triple-negative breast cancer cells. Breast Cancer Res. 21, 104 (2019).
-
Chen, X. et al. Targeting replicative stress and DNA repair by combining PARP and Wee1 kinase inhibitors is synergistic in triple negative breast cancers with cyclin E or BRCA1 alteration. Cancers 13, 1656 (2021).
-
Heidler, C. L. et al. Prexasertib (LY2606368) reduces clonogenic survival by inducing apoptosis in primary patient-derived osteosarcoma cells and synergizes with cisplatin and talazoparib. Int. J. Cancer 147, 1059–1070 (2020).
-
Garcia, T. B. et al. A small-molecule inhibitor of WEE1, AZD1775, synergizes with olaparib by impairing homologous recombination and enhancing DNA damage and apoptosis in acute leukemia. Mol. Cancer Ther. 16, 2058–2068 (2017).
-
Karnak, D. et al. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin. Cancer Res. 20, 5085–5096 (2014).
-
Smith, H. L., Prendergast, L. & Curtin, N. J. Exploring the synergy between PARP and CHK1 inhibition in matched BRCA2 mutant and corrected cells. Cancers 12, 878 (2020).
-
Krebs, M. G. et al. Abstract CT026: Phase I study of AZD6738, an inhibitor of ataxia telangiectasia Rad3-related (ATR), in combination with olaparib or durvalumab in patients (pts) with advanced solid cancers. Cancer Res. 78 (Suppl. 13), CT026 (2018).
-
Wethington, S. L. et al. Combination of PARP and ATR inhibitors (olaparib and ceralasertib) shows clinical activity in acquired PARP inhibitor-resistant recurrent ovarian cancer. J. Clin. Oncol. 39, 5516 (2021).
-
Westin, S. N. et al. EFFORT: EFFicacy Of adavosertib in parp ResisTance: a randomized two-arm non-comparative phase II study of adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. J. Clin. Oncol. 39, 5505 (2021). This is the first clinical study to show the activity of the WEE1 inhibitor adavosertib in which all patients presented PARPi-resistant ovarian cancer.
-
Do, K. T. et al. Immune modulating activity of the CHK1 inhibitor prexasertib and anti-PD-L1 antibody LY3300054 in patients with high-grade serous ovarian cancer and other solid tumors. Cancer Immunol. Immunother. 70, 2991–3000 (2021).
-
Kwon, M. et al. Phase II study of ceralasertib (AZD6738), in combination with durvalumab in patients with metastatic melanoma who have failed prior anti-PD-1 therapy. J. Clin. Oncol. 39, 9514 (2021). This study shows the most promising clinical result of the combination of an ATRi with anti-PD1/PDL1 therapy.
-
Li, F. et al. mTOR inhibition overcomes primary and acquired resistance to Wee1 inhibition by augmenting replication stress in epithelial ovarian cancers. Am. J. Cancer Res. 10, 908–924 (2020).
-
Hai, J. et al. Synergy of WEE1 and mTOR inhibition in mutant KRAS-driven lung cancers. Clin. Cancer Res. 23, 6993–7005 (2017).
-
Wu, S. et al. Activation of WEE1 confers resistance to PI3K inhibition in glioblastoma. NeuroOncol. 20, 78–91 (2018).
-
Wu, W. et al. Combination of the Chk1 inhibitor (prexasertib) with a PI3K/mTOR inhibitor (LY3023414) induces synergistic anti-tumor activity in triple negative breast cancer (TNBC) models. Cancer Res. 79 (Suppl. 13), Abstr. 3508 (2019).
-
Ding, Y. et al. PI3K/AKT signaling pathway is transcriptionally elevated in prexasertib-resistant TNBC PDX models. Cancer Res. 78 (Suppl. 13), Abstr. 2586 (2018).
-
Song, X. et al. Synergistic targeting of CHK1 and mTOR in MYC-driven tumors. Carcinogenesis 42, 448–460 (2021).
-
Nayak, S., Calvo, J. A. & Cantor, S. B. Targeting translesion synthesis (TLS) to expose replication gaps, a unique cancer vulnerability. Expert Opin. Ther. Targets 25, 27–36 (2021).
-
Lee, J. W. et al. Combined Aurora kinase A (AURKA) and WEE1 inhibition demonstrates synergistic antitumor effect in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 25, 3430–3442 (2019).
-
Zhou, L. et al. A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations. Leukemia 29, 807–818 (2015).
-
Schwartz, J. et al. Synergistic anti-leukemic interactions between ABT-199 and panobinostat in acute myeloid leukemia ex vivo. Am. J. Transl. Res. 8, 3893–3902 (2016).
-
Wang, G. et al. Synergistic antitumor interactions between MK-1775 and panobinostat in preclinical models of pancreatic cancer. Cancer Lett. 356, 656–668 (2015).
-
de Jong, M. R. W. et al. WEE1 inhibition enhances anti-apoptotic dependency as a reult of premature mitotic entry and DNA damage. Cancers 11, 1743 (2019).
-
Nojima, H. et al. Differential properties of mitosis-associated events following CHK1 and WEE1 inhibitor treatments in human tongue carcinoma cells. Exp. Cell Res. 386, 111720 (2020).
-
Caeser, R. & Sen, T. Should WEE(1) CHK(1) in on the FAM(122A)ily? Mol. Cell 80, 377–378 (2020).
-
Li, F. et al. CHK1 inhibitor blocks phosphorylation of FAM122A and promotes replication stress. Mol. Cell 80, 410–422.e6 (2020).
-
Nam, A. R. et al. Inhibition of ATR increases the sensitivity to WEE1 inhibitor in biliary tract cancer. Cancer Res. Treat. 52, 945–956 (2020).
-
Bukhari, A. B. et al. Inhibiting Wee1 and ATR kinases produces tumor-selective synthetic lethality and suppresses metastasis. J. Clin. Invest. 129, 1329–1344 (2019).
-
Restelli, V. et al. DNA damage response inhibitor combinations exert synergistic antitumor activity in aggressive B-cell lymphomas. Mol. Cancer Ther. 18, 1255–1264 (2019).
-
Qi, W. et al. Inhibition of Wee1 sensitizes AML cells to ATR inhibitor VE-822-induced DNA damage and apoptosis. Biochem. Pharmacol. 164, 273–282 (2019).
-
Deneka, A. Y. et al. Synthetic lethal targeting of mitotic checkpoints in HPV-negative head and neck cancer. Cancers 12, 306 (2020).
-
Di Rorá, A. G. L. et al. Synergism through WEE1 and CHK1 inhibition in acute lymphoblastic leukemia. Cancers 11, 1654 (2019).
-
Maya-Mendoza, A. et al. Myc and Ras oncogenes engage different energy metabolism programs and evoke distinct patterns of oxidative and DNA replication stress. Mol. Oncol. 9, 601–616 (2015).
-
Cottini, F. et al. Synthetic lethal approaches exploiting DNA damage in aggressive myeloma. Cancer Discov. 5, 972–987 (2015).
-
Murga, M. et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat. Struct. Mol. Biol. 18, 1331–1335 (2011).
-
Liu, J. F. et al. Phase II study of the WEE1 inhibitor adavosertib in recurrent uterine serous carcinoma. J. Clin. Oncol. 39, 1531–1539 (2021).
-
Al Zubaidi, T. et al. Targeting the DNA replication stress phenotype of KRAS mutant cancer cells. Sci. Rep. 11, 3656 (2021).
-
Konstantinopoulos, P. A. et al. A replication stress biomarker is associated with response to gemcitabine versus combined gemcitabine and ATR inhibitor therapy in ovarian cancer. Nat. Commun. 12, 5574 (2021). This study tests several biomarkers of replication stress in patient tumour samples from a randomized clinical trial. It proposes a score to identify tumours that are more sensitive to replication stress-inducing agents.
-
Guerrero Llobet, S. et al. An mRNA expression-based signature for oncogene-induced replication-stress. Oncogene 41, 1216–1224 (2022).
-
Cleary, J. M., Aguirre, A. J., Shapiro, G. I. & D’Andrea, A. D. Biomarker-guided development of DNA repair inhibitors. Mol. Cell 78, 1070–1085 (2020).
-
Dunlop, C. R. et al. Complete loss of ATM function augments replication catastrophe induced by ATR inhibition and gemcitabine in pancreatic cancer models. Br. J. Cancer 123, 1424–1436 (2020).
-
Perkhofer, L. et al. ATM deficiency generating genomic instability sensitizes pancreatic ductal adenocarcinoma cells to therapy-induced DNA damage. Cancer Res. 77, 5576–5590 (2017).
-
Schmitt, A. et al. ATM deficiency is associated with sensitivity to PARP1- and ATR inhibitors in lung adenocarcinoma. Cancer Res. 77, 3040–3056 (2017).
-
Min, A. et al. AZD6738, A novel oral inhibitor of ATR, induces synthetic lethality with ATM deficiency in gastric cancer cells. Mol. Cancer Ther. 16, 566–577 (2017).
-
Wang, Y., Hoang, L., Ji, J. X. & Huntsman, D. G. SWI/SNF complex mutations in gynecologic cancers: molecular mechanisms and models. Annu. Rev. Pathol. Mech. Dis. 15, 467–492 (2020).
-
Damelin, M. & Bestor, T. H. The decatenation checkpoint. Br. J. Cancer 96, 201–205 (2007).
-
Dykhuizen, E. C. et al. BAF complexes facilitate decatenation of DNA by topoisomerase IIα. Nature 497, 624–627 (2013).
-
Williamson, C. T. et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat. Commun. 7, 13837 (2016).
-
Flynn, R. L. et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 347, 273–277 (2015).
-
Heaphy, C. M. et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 179, 1608–1615 (2011).
-
Laroche-Clary, A. et al. ATR inhibition broadly sensitizes soft-tissue sarcoma cells to chemotherapy independent of alternative lengthening telomere (ALT) status. Sci. Rep. 10, 7488 (2020).
-
Deeg, K. I., Chung, I., Bauer, C. & Rippe, K. Cancer cells with alternative lengthening of telomeres do not display a general hypersensitivity to ATR inhibition. Front. Oncol. 6, 186 (2016).
-
Lewis, C. W. et al. Upregulation of MyT1 promotes acquired resistance of cancer cells to WEE1 inhibition. Cancer Res. 79, 5971–5985 (2019).
-
Tsai, S. et al. ARID1A regulates R-loop associated DNA replication stress. PLoS Genet. 17, e1009238 (2021).
-
Oku, Y. et al. Augmentation of the therapeutic efficacy of WEE1 kinase inhibitor AZD1775 by inhibiting the YAP-E2F1-DNA damage response pathway axis. FEBS Open. Bio 8, 1001–1012 (2018).
Replication stress for cancer therapy