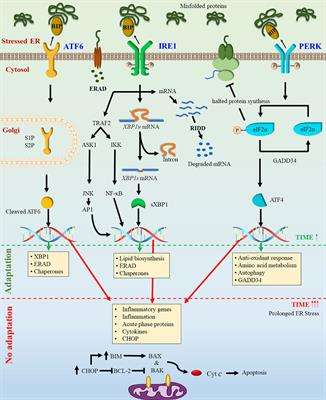
Figure 1.Endoplasmic Reticulum (ER) homeostasis/stress and the unfolded protein response (UPR) signaling in physiopathologic conditions. The UPR consists of three signaling pathways initiated by detachment of upstream transducers activating transcription factor 6 (ATF6), protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) and inositol-requiring enzyme 1 (IRE1) from glucose-regulated protein 78 (GRP78), a chaperone protein that monitors accumulation of unfolded and misfolded proteins inside the ER lumen. (A) In physiological (unstressed) states, these transducers bind to the folding chaperone GRP78 and keep the ER quiescent; (B) ER stress inducers accumulate unfolded/misfolded proteins in the ER lumen by impairing protein folding. Higher GRP78 affinity for unfolded/misfolded proteins dissociates GRP78 from ATF6, PERK and IRE1, enabling GRP78 unfolded/misfolded protein binding that then initiates three UPR signaling cascades. Specifically: (1) ATF6 signaling involves its translocation to Golgi apparatus for proteolytic cleavage by site-1 protease (S1P) and site-2 protease (S2P) and subsequent release into the nucleus as an active transcription factor to induce expression of GRP78, ubiquitously expressed X-box binding protein 1 (XBP1u) etc.; (2) PERK signaling consist of auto-phosphorylation of PERK (P-PERK), generating an active kinase that phosphorylates eukaryotic translation-initiation factor 2α (P-eIF2α). P-eIF2α blocks its translation initiating activity and induces ATF4 phosphorylation (P-ATF4) leading to P-ATF4 nuclear translocation as a transcription factor to induce expression of GRP78, C/EBP homologous protein (CHOP), XBPu etc.; (3) IRE1α signaling includes IRE1α phosphorylation (P-IRE1α), an active endonuclease that cleaves XBP-1u mRNA to XBP-1s mRNA, which is then translated to an active transcription factor to induce UPR target genes encoding GRP78, ERAD proteins etc. Thus, by increasing ER chaperone protein levels and blocking of eIF2α-mediated protein synthesis, UPR signaling adjusts cells to increased ER stress conditions by transporting excess unfolded/misfolded proteins to ERAD-complex for proteasome-mediated degradation, thereby re-establishing ER homeostasis and sustaining cell survival, whereas prolonged and/or severe ER stress induces apoptosis by CHOP activation, ER-linked caspase 12-mediated caspase 3 cleavage and/or ER Ca2+ efflux associated mitochondrial cytochrome-c release.
Figure 1.Endoplasmic Reticulum (ER) homeostasis/stress and the unfolded protein response (UPR) signaling in physiopathologic conditions. The UPR consists of three signaling pathways initiated by detachment of upstream transducers activating transcription factor 6 (ATF6), protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) and inositol-requiring enzyme 1 (IRE1) from glucose-regulated protein 78 (GRP78), a chaperone protein that monitors accumulation of unfolded and misfolded proteins inside the ER lumen. (A) In physiological (unstressed) states, these transducers bind to the folding chaperone GRP78 and keep the ER quiescent; (B) ER stress inducers accumulate unfolded/misfolded proteins in the ER lumen by impairing protein folding. Higher GRP78 affinity for unfolded/misfolded proteins dissociates GRP78 from ATF6, PERK and IRE1, enabling GRP78 unfolded/misfolded protein binding that then initiates three UPR signaling cascades. Specifically: (1) ATF6 signaling involves its translocation to Golgi apparatus for proteolytic cleavage by site-1 protease (S1P) and site-2 protease (S2P) and subsequent release into the nucleus as an active transcription factor to induce expression of GRP78, ubiquitously expressed X-box binding protein 1 (XBP1u) etc.; (2) PERK signaling consist of auto-phosphorylation of PERK (P-PERK), generating an active kinase that phosphorylates eukaryotic translation-initiation factor 2α (P-eIF2α). P-eIF2α blocks its translation initiating activity and induces ATF4 phosphorylation (P-ATF4) leading to P-ATF4 nuclear translocation as a transcription factor to induce expression of GRP78, C/EBP homologous protein (CHOP), XBPu etc.; (3) IRE1α signaling includes IRE1α phosphorylation (P-IRE1α), an active endonuclease that cleaves XBP-1u mRNA to XBP-1s mRNA, which is then translated to an active transcription factor to induce UPR target genes encoding GRP78, ERAD proteins etc. Thus, by increasing ER chaperone protein levels and blocking of eIF2α-mediated protein synthesis, UPR signaling adjusts cells to increased ER stress conditions by transporting excess unfolded/misfolded proteins to ERAD-complex for proteasome-mediated degradation, thereby re-establishing ER homeostasis and sustaining cell survival, whereas prolonged and/or severe ER stress induces apoptosis by CHOP activation, ER-linked caspase 12-mediated caspase 3 cleavage and/or ER Ca2+ efflux associated mitochondrial cytochrome-c release.
Figure 2.Endometrial regulation and role of ER stress during menstrual cycle and in endometriosis. Human endometrium undergoes several cellular, molecular and morphologic changes during menstrual cycle. (A) In situ studies demonstrate increased GRP78 expression during late secretory and early proliferative phases, which may be result from low estradiol (E2) levels and/or pro-inflammatory milieu. In culture, E2 blocks ER stress (tunicamycin)-induced GRP78 expression supporting a role for E2 in favor of ER homeostasis by suppressing GRP78 (ER stress sensor) during the E2 dominated phases of the cycle, which directly or indirectly contributes to angiogenesis, cell proliferation/apoptosis and protein secretion occurring each cycle; (B) Alternatively, significantly high GRP78 levels in ectopic endometriotic tissues may result from increased local E2 production, aberrant sex steroid signaling (progesterone resistance) and/or an enhanced increased inflammatory milieus. These severe/prolonged conditions may activate ER stress/UPR signaling cascades to enhance VEGF expression and induce angiogenesis, required for endometriotic tissue growth. Moreover, significant reduction in estrogenic response in severe ER stress (tunicamycin) condition may contribute to aberrant steroid response in endometriotic tissues.
Figure 2.Endometrial regulation and role of ER stress during menstrual cycle and in endometriosis. Human endometrium undergoes several cellular, molecular and morphologic changes during menstrual cycle. (A) In situ studies demonstrate increased GRP78 expression during late secretory and early proliferative phases, which may be result from low estradiol (E2) levels and/or pro-inflammatory milieu. In culture, E2 blocks ER stress (tunicamycin)-induced GRP78 expression supporting a role for E2 in favor of ER homeostasis by suppressing GRP78 (ER stress sensor) during the E2 dominated phases of the cycle, which directly or indirectly contributes to angiogenesis, cell proliferation/apoptosis and protein secretion occurring each cycle; (B) Alternatively, significantly high GRP78 levels in ectopic endometriotic tissues may result from increased local E2 production, aberrant sex steroid signaling (progesterone resistance) and/or an enhanced increased inflammatory milieus. These severe/prolonged conditions may activate ER stress/UPR signaling cascades to enhance VEGF expression and induce angiogenesis, required for endometriotic tissue growth. Moreover, significant reduction in estrogenic response in severe ER stress (tunicamycin) condition may contribute to aberrant steroid response in endometriotic tissues.
Figure 3.Regulation and therapeutic targeting of ER stress in reproductive tissue cancers. Several reproductive tissue cancers display increased ER stress chaperone GRP78 and the UPR signaling proteins ATF6, PERK and IRE levels in situ, indicating increased ER stress with a cancer cell specific adaptation to the stress condition via increased autophagy activities to degrade unfolded/misfolded protein as well as enhanced ER protein folding capacity, which result in ER homeostasis, thereby supporting growth and invasion of tumor cells. Several studies reported that disruption of cancer cell specific adaption to severe/prolong ER stress by chemotherapeutic agents (cisplatin, paclitaxel) or naturally occurring agents (curcumin, resveratrol etc.) can reduce cancer cell proliferation and invasion and increase apoptosis, resulting in tumor growth regression.
Figure 3.Regulation and therapeutic targeting of ER stress in reproductive tissue cancers. Several reproductive tissue cancers display increased ER stress chaperone GRP78 and the UPR signaling proteins ATF6, PERK and IRE levels in situ, indicating increased ER stress with a cancer cell specific adaptation to the stress condition via increased autophagy activities to degrade unfolded/misfolded protein as well as enhanced ER protein folding capacity, which result in ER homeostasis, thereby supporting growth and invasion of tumor cells. Several studies reported that disruption of cancer cell specific adaption to severe/prolong ER stress by chemotherapeutic agents (cisplatin, paclitaxel) or naturally occurring agents (curcumin, resveratrol etc.) can reduce cancer cell proliferation and invasion and increase apoptosis, resulting in tumor growth regression.
Figure 4.Role of ER stress in spermatogenesis. Increased protein synthesis and/or degradation to compensate intracellular, membranous, biochemical and structural changes during spermatogenesis assign a central role to the ER in coordinating these events. Several reports indicate that testicular hyperthermia induces UPR signaling cascades suggesting that increased ER stress may impair spermatogenesis. Endocrine-disrupting chemicals bisphenol-A and diethylstilbestrol and cadmium, an environmental toxicant, are reported to cause severe ER stress by elevating IRE1α phosphorylation and CHOP expression in spermatozoa. The resulting increased CHOP expression then triggers apoptosis via activating caspase 3 in sperm, which may reduce or eliminate fertilization capacity.
Figure 4.Role of ER stress in spermatogenesis. Increased protein synthesis and/or degradation to compensate intracellular, membranous, biochemical and structural changes during spermatogenesis assign a central role to the ER in coordinating these events. Several reports indicate that testicular hyperthermia induces UPR signaling cascades suggesting that increased ER stress may impair spermatogenesis. Endocrine-disrupting chemicals bisphenol-A and diethylstilbestrol and cadmium, an environmental toxicant, are reported to cause severe ER stress by elevating IRE1α phosphorylation and CHOP expression in spermatozoa. The resulting increased CHOP expression then triggers apoptosis via activating caspase 3 in sperm, which may reduce or eliminate fertilization capacity.
Figure 5.Regulation and the impact of ER stress/UPR signaling cascades during oogenesis and preimplantation. During folliculogenesis, enhanced IRE1α, PERK, GRP78 and XPB1s levels in the granulosa cells of large secondary follicles and later stages as well as elevated XBP1s levels in cumulus cells from fertilized oocytes indicate physiological involvement of UPR signaling during oogenesis and fertilization. However, increased fatty acid levels and obesity can dysregulate protein secretion and mitochondrial activity by impairing ER homeostasis, causing abnormal embryonic development. Salubrinal treatment of cumulus-oocyte complex maintains normal preimplantation embryo development by reversing these conditions. The preimplantation embryo also requires extensive protein synthesis for proper development and implantation. Severe ER stress induced by tunicamycin in 2-cell stage embryos does not affect development until the end of morula stage. However, severe ER stress impairs blastocyst formation via extensive apoptosis. Use of TUDCA completely reverses these negative effects, indicating that ER homeostasis is crucial during blastocyst formation and subsequent development.
Figure 5.Regulation and the impact of ER stress/UPR signaling cascades during oogenesis and preimplantation. During folliculogenesis, enhanced IRE1α, PERK, GRP78 and XPB1s levels in the granulosa cells of large secondary follicles and later stages as well as elevated XBP1s levels in cumulus cells from fertilized oocytes indicate physiological involvement of UPR signaling during oogenesis and fertilization. However, increased fatty acid levels and obesity can dysregulate protein secretion and mitochondrial activity by impairing ER homeostasis, causing abnormal embryonic development. Salubrinal treatment of cumulus-oocyte complex maintains normal preimplantation embryo development by reversing these conditions. The preimplantation embryo also requires extensive protein synthesis for proper development and implantation. Severe ER stress induced by tunicamycin in 2-cell stage embryos does not affect development until the end of morula stage. However, severe ER stress impairs blastocyst formation via extensive apoptosis. Use of TUDCA completely reverses these negative effects, indicating that ER homeostasis is crucial during blastocyst formation and subsequent development.
Table 1.Regulation and potential impact of endoplasmic reticulum (ER) stress molecules during normal and abnormal pregnancy conditions according to current literature.
Table 1.Regulation and potential impact of endoplasmic reticulum (ER) stress molecules during normal and abnormal pregnancy conditions according to current literature.
ER Stress MoleculesAlteration/SourcesAction/Significance/AssociationPregnancy Stage/GroupsHSC70Increased secretion from blastocystParacrine action for proper folding of newly translated and misfolded proteins in decidual cellsImplantation window [133]GRP78Increased in endometrial stromal cellsRecurrent miscarriageImplantation window [114]IRE1αKnock-out mouse
Smaller placenta and embryo sizesA reduced VEGF-A levels in the placenta as well as severe dysfunction of the labyrinth placentaPlacentation in mouse [157]GRP78 and VCPDown-regulation in decidual cellsActs with oxidative stress as cofactor for molecular induction of early pregnancy lossSpecimens from Early pregnancy loss [159]GRP78, P-eIF2α and XBP-1Increased levels in syncytiotrophoblastsIncreased ER stress during normal laborLabor vs. Non-labor placentas [154]GRP78, IRE1 and XBP-1s-In fetal membranes and myometrium
-LPS mediated increase in explant cultures of fetal membranes and myometrium- Increased ER stress in preterm and term labor
– Infection may induce ER stressTerm and spontaneous pre-term labor vs. non-labor placenta specimens. Fetal membranes and myometrium from non-laboring women at the time of term Cesarean section [155]GRP78, P-eIF2α, ATF4, and CHOPIncreased in placentaElevated ER stress and deregulation of proper protein folding during pregnancyDuring pregnancy in rat [160]P-eIF2αIncreased in placentaIncreased ER stress that reduce placental protein synthesisFGR (GA weeks 28–38) vs. term control (GA weeks 39–40) [161]GRP78 and 94, P-PERK, eIF2a, P-eIF2a, XBP1, CHOP, IRE1, P-IRE1Elevated levels in placentasExaggerated ER stress in preeclampsiaPreeclamptic (mean GA weeks 33.6) vs. control placentas (mean GA weeks 39.2) [162]UPR transcription factors ATF4, ATF6α and ATF6βIncreased nuclear localization in the syncytiotrophoblastsIncreased ER stress and contributes to reduced PlGF protein levelsPreeclamptic placentas (GA < 34 weeks ) vs. term control [163]PERK-induced p-eIF2α, ATF6 and XBP1uIncreased levels in extra-villous trophoblasts, decidual cells and macrophagesIncreased ER stress may impair placental growth associated with FGR and FGR + pre-eclampsiaDecidual tissues from FGR (mean GA weeks 31.9) or FGR with pre-eclampsia (mean GA weeks 30.3) vs. term control (mean GA weeks 38.7) [138]P-IRE1α, ATF6, XBP-1, GRP78 and GRP94Increased in placental lysatesImpaired ER stress may cause placental dysfunction that triggers preeclampsia Early-onset (<34 weeks) pre-eclampsia vs. late-onset pre-eclampsia and normotensive controls [164]P-eIF2α, eIF2α, XBP-1 and GRP78Increased in placental lysatesAssociation between increased ER stress and preterm laborSpontaneous pre-term placentas (due to acute chorioamnionitis and other conditions) vs. term controls [164]